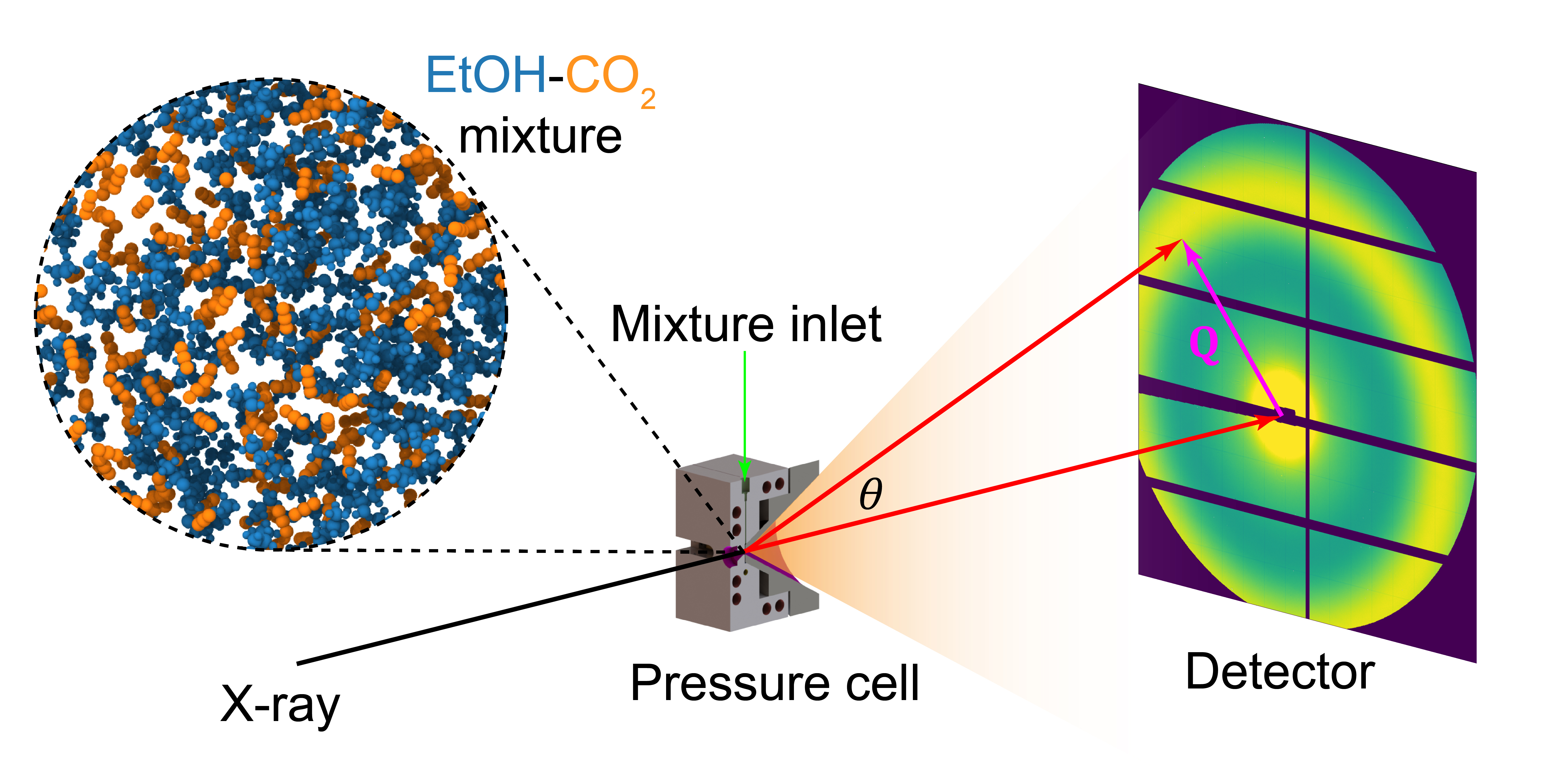
Supercritical fluids (SCFs) are often regarded as homogeneous at the microscopic level, but our studies reveal a different picture: even well-mixed supercritical systems can exhibit hidden structural heterogeneities. Using combined experimental and simulation techniques, we show that supercritical mixtures can undergo spontaneous microscopic phase separation, driven by the formation of energetically favorable clusters. This challenges the classical notion of full miscibility in SCFs and has far-reaching implications for applications in separations, catalysis, and materials synthesis, where nanoscale fluid structure governs macroscopic performance.
In our recent work, we examined both single-component and binary supercritical systems. For pure fluids, we discovered that even above the critical point, transient clusters form that locally resemble a liquid-like phase, initiating a kinetic pathway toward phase separation without any macroscopic interface (Fan et al., 2024). Extending this to mixtures, we studied ethanol–CO2 using synchrotron X-ray scattering and molecular dynamics simulations, finding persistent ethanol-rich clusters stabilized by hydrogen bonding (Fan et al., 2025). These observations demonstrate that microscopic immiscibility and cluster dynamics are general features of supercritical fluids, governed by a competition between energetic attractions and entropic mixing.
References
2025
-
Supercritical Ethanol–CO2 Mixtures Exhibit Microscopic Immiscibility: A Combined Study Using X-ray Scattering and Molecular Dynamics Simulations
Jingcun Fan, Taekeun Yoon, Guillaume Vignat, Haoyuan Li, Khaled Younes, Arijit Majumdar, Priyanka Muhunthan, Dimosthenis Sokaras, Thomas Weiss, Ivan Rajkovic, and Matthias Ihme
The Journal of Physical Chemistry Letters, 2025
PMID: 40604336
Supercritical mixtures of ethanol (EtOH) and carbon dioxide (CO2) are classified as type-I mixtures, with complete macroscopic miscibility. However, differences in molecular polarity and interactions suggest a distinct phase behavior at the microscopic level. Here, we combine small angle X-ray scattering experiments and molecular dynamics (MD) simulations to investigate the microscopic structure of EtOH–CO2 mixtures under supercritical conditions. The structure factor exhibits nonlinear composition-dependent behavior, revealing pronounced local density fluctuations. The complementary MD simulations, using optimized force field parameters, provide atomistic insight, showing that EtOH forms self-associated, hydrogen-bonded aggregates, while CO2 remains more uniformly distributed. Cluster analysis identifies a preferential EtOH-rich composition exceeding the bulk average, governed by a balance between energetic and entropic competition. These results demonstrate that, contrary to macroscopic expectations, the mixture exhibits significant microscopic heterogeneity and immiscibility, which may influence solubility, reactivity, transport properties, and thermodynamic response functions. These findings challenge the conventional views of type-I fluids and emphasize the necessity of revising mixture states and considering molecular polarity.
2024
-
Heterogeneous Cluster Energetics and Nonlinear Thermodynamic Response in Supercritical Fluids
Jingcun Fan, Nguyen Ly, and Matthias Ihme
Physical Review Letters, Dec 2024